Redesigning the Sentimag® surgical guidance system to transform breast cancer care
Background
The partnership between Endomag and eg technology signifies a collaborative effort to address the pressing challenges in breast cancer diagnosis and treatment. By combining Endomag’s expertise in magnetic guidance technology with eg technology’s proficiency in product design and engineering, the two companies have created a synergy that has led to groundbreaking innovations.
Furthermore, the third-generation Sentimag® unit not only represents a significant advancement in breast cancer surgery equipment but also serves as a testament to the power of interdisciplinary collaboration in healthcare innovation. This partnership exemplifies a shared commitment to advancing medical technology for the benefit of patients worldwide, underscoring the importance of collaboration and innovation in the fight against breast cancer.
More than 450,000 patients have been treated using the Sentimag® Platform
Redefining the redesign
The next generation Sentimag® device needed to detect both Magseed® – a tiny, inert, surgical grade magnetic marker, and Magtrace® – a suspension of iron nano-particles that migrates through the lymphatic system. In addition to improved system performance and extra detection functionality, key requirements were to revolutionise the aesthetics and User Interface (UI) of the system and to redesign the internal electromechanical assembly to be easy to assemble, robust and reliable. Successfully executing these requirements would enable an improved user experience, higher volume manufacture, commercial scale-up and access to global markets.
Each year 2 million women are diagnosed with breast cancer and most will undergo some form of surgery
Delivering complexity in a simple way
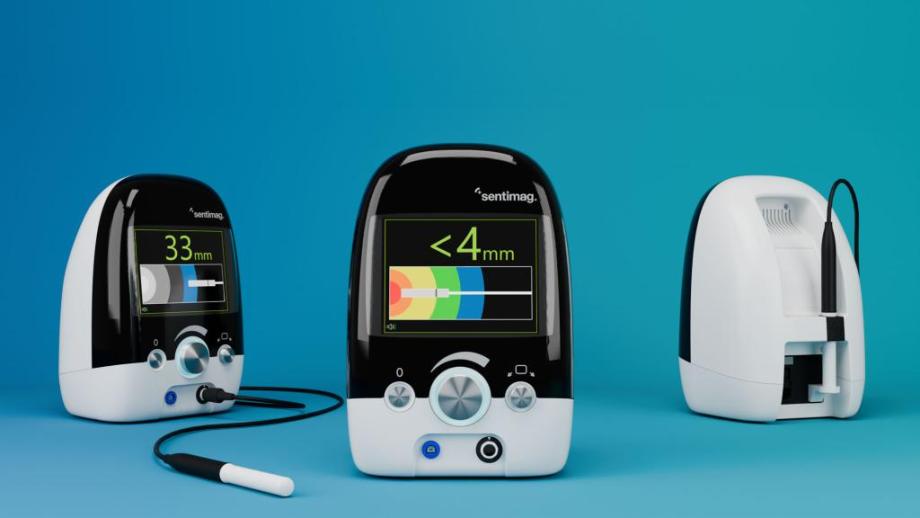
Used by surgeons to determine the location and proximity of the Sentimag® probe relative to Magseed® and Magtrace®, presenting complex signal processing in a simple and intuitive way was always at the heart of the Sentimag® redesign.
eg technology are experts in developing electromechanical devices so we knew that our industrial design, mechanical engineering, electronics and software teams had to work together closely to ensure efficient product development. The foundations for a successful medical device development programme are detailed user and product specifications and along with these, the technical performance requirements were defined by undertaking extensive usability analysis with end users and assembly partners. Understanding the requirements of all stakeholders was key to unlocking the potential to redesign Sentimag® and set a new standard in the performance and design of a medical device.
Once the user and product requirements were defined, a range of aesthetic concepts were created.
Endomag products are now routinely used by over 1000 hospitals in more than 45 countries
Designing the Solution
It was clear from the initial concept development that in order to maximise the user experience, the design emphasis of Sentimag® should be around a large, clear, UI. Our industrial designers and software engineers worked closely to develop a UI that had intuitive and informative visuals and audio, accurately conveying the location of the probe and how it moves in an unambiguous way, meaning the surgeon can operate with full confidence. The large, monolithic, colour-isolated front fascia offers a borderless centrepiece to the unit. The screen emanates vibrantly through the fascia and a simple set of input dials, bold colour breaks and minimal connections complete the uncomplicated and modern aesthetic. When the surgeon wants quantitative clarification of the probe location they can rapidly focus on the screen from a distance and read the numbers without confusion or distraction.
Portability and ease of setup prior to surgery were also of prime importance to our product designers, so we used our cross-disciplinary engineering knowledge to deliver a solution to Endomag that met the brief.
Our electronics team carried out extensive development on the unit’s FPGA, processing large amounts of data in real time to convert complex magnetic field measurements into visual and audio indications to guide the surgeon during a procedure. Our electronics team worked alongside our mechanical engineers to design a chassis that met EMC requirements, whilst also being easy to manufacture, assemble and service. It was essential that the external dials and connectors aligned perfectly in the casework, so our designers analysed tolerances and designed compliance into assemblies to eliminate the need for unrealistically accurate components.
Prototypes were produced throughout the project to de-risk key aspects of the development process. After iterating through proof-of-principle, alpha and beta prototypes, high-quality, pre-production prototypes were made to fully test all aspects of the device. Verification and validation of the system was done in close collaboration with the Endomag team to confirm technical performance along with summative evaluation with end users. Our understanding of manufacturing and assembly processes meant that we naturally considered how assembly sequences, robustness and tolerances would be accommodated.
A variety of prototyping methods were used across the development process to de-risk different elements of the design
Regulations and device classification
The extensive electronics and software engineering was carried out in accordance with IEC 60601 and IEC 62304 respectively, with the core elements of the software for Sentimag® being classified as software safety class C. This is the highest level for medical devices.
The full project was developed within our ISO 13485 quality system with full risk management including in-use and human error formally documented in accordance with ISO 14971.
The ultimate aim for Endomag was for the device to achieve global regulatory approvals with FDA clearance for the updated device being granted in 2023.
End Result
We worked closely with the Endomag engineering team, supporting them throughout the multiphase programme. We delivered pre-production prototypes, supported transfer to manufacture and provided the required design and technical documentation for Endomag to incorporate into their technical file. The Sentimag® base unit is now able to detect and process data from the Magseed®, MagseedPro® and Magtrace® in clever ways, giving distinct signals between Magtrace® and MagseedPro® when switching between the device’s ‘Counts’ and ‘Measure’ modes, giving surgeon’s intuitive proximity and distance readings on the large display. The Sentimag® is not only practical through easy cleaning and portability but the highly-considered design looks premium, high quality and stands out in its clinical setting.
In addition to the full Sentimag® development, we worked with Endomag to improve the assembly procedure and reliability of the probe which demonstrates how we can quickly get up to speed and offer engineering support in a smaller capacity.
Endomag announced in 2023 that it had received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) for the 3rd generation Sentimag® to be used with Magtrace®, making it the first and only non-radioactive solution approved in the U.S. that can perform lesion localisation and sentinel node biopsy.
For more information, please visit the eg technology website here: www.egtechnology.co.uk