At Team Consulting, we have been helping our clients develop elegant solutions to complex healthcare challenges for 37 years. One of the key challenges in this space is how to cost effectively innovate in the highly constrained and regulated world of MedTech product design. It is necessary to balance requirements and constraints related to commercial viability, desirability and technical feasibility. Simply put, your product will not be successful if there is no clear market need, if it is not attractive to users and if you are unable to engineer the necessary levels of performance and reliability at an appropriate cost and to a level appropriate for regulatory compliance. Achieving a harmonious balance with these three factors involves a careful process of ‘design thinking’ throughout your development.
What is design thinking?
Design thinking is about understanding the competing design constraints in your product and using a framework to evaluate and balance them. When applied to device development, it enables you to deliver medical products that are not only safe and effective, but loved by their end users.
Before describing our approach to design thinking in medical devices, it is important to first understand the non-negotiable legislative constraints.
Legislative constraints
For any product to be marketable, it must first comply with all applicable legislation in the territory where it is to be marketed. In general, the more safety critical a product, the greater the regulatory obligations and rigour, traceability and control needed to achieve conformity with those regulations.
To put this in perspective, the UK’s General Product Safety Regulation (2005), which governs the marketing and distribution of consumer products, comprises 30 pages of requirements and obligations for manufacturers. In comparison, the Medical Devices Directive (currently transposed into UK law) contains 60 pages of articles and annexes. The new Medical Devices Regulation (“MDR”) has over 200 pages of requirements and obligations.
At the highest level, the primary intent of medical device regulations and standards is to ensure that clinical benefit is delivered to patients and users at an appropriate level of residual risk. Quality management processes ensure that corrective and preventative actions (CAPA) are taken whenever medical devices cause, or risk, previously unforeseen harm to patients, users or the environment.
As an ongoing process, this CAPA approach ensures a continuous ‘raising of the bar’ in regard to judging acceptable risk. As a result, medical device manufacturers face an ever increasing workload to be able to declare that any residual risks associated with the intended use of their medical product are reduced to as low a level as possible and are at least commensurate with the current state of the art. Medical device manufacturers must seek to innovate in this space.
Applying design thinking
If you are new to the concept of design thinking, Tim Brown's“Change by Design – how design thinking transforms organisation and inspires innovation”, is a great place to start. Brown describes the foundation of design thinking as “the willing and even enthusiastic acceptance of competing constraints”, stating that “the first stage of the design process is often about discovering which constraints are important and establishing a framework for evaluating them.” Brown describes three types of constraints:
- Commercial viability – what is likely to become part of a sustainable business model?
- Desirability/usability – what makes sense to people and for people?
- Technical feasibility – what is functionally possible within the foreseeable future?
These constraints provide a useful framework to explore Team’s approach to design thinking and innovation in the MedTech product design space.
Commercial viability
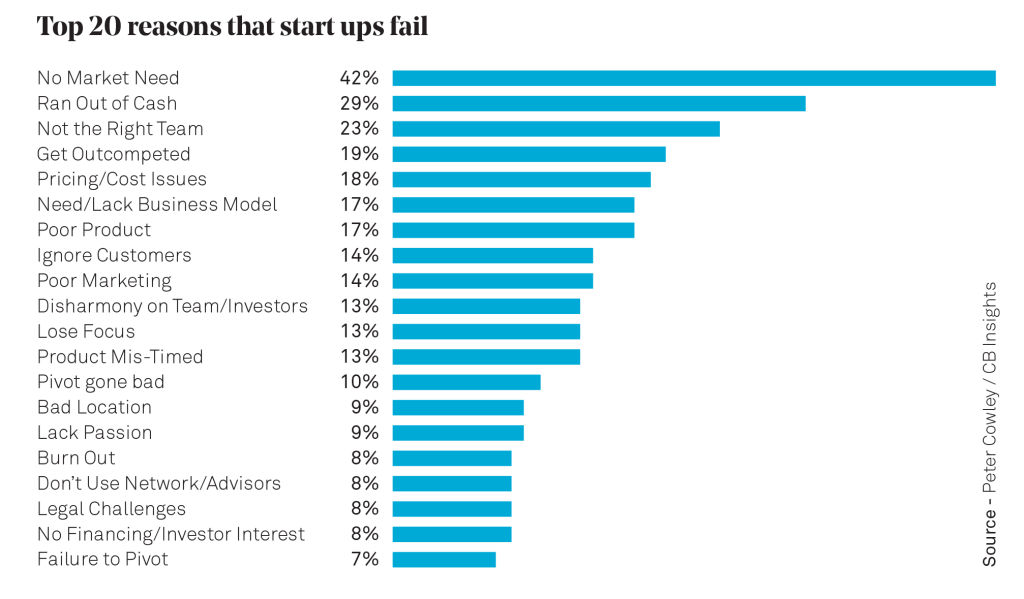
Commercial viability focuses on key attributes needed to achieve a sustainable business model in the healthcare sector. To help think about this, it is good to initially understand why some business models are not sustainable. The diagram below provides very useful guidance for early stage MedTech start-ups. It shows that the number one reason start-ups fail is a lack of market need. With primary pitfalls in mind, we can start to consider the tools that are available to avoid them.
Front End Innovation
One of the key ways Team helps its clients ensure commercial viability is through the application of an effective Front End Innovation (FEI) process. FEI is an agile, cost-effective process that involves conducting early stage research and setting a unified vision for your company and product goals. It includes creating an initial set of product needs (termed the Target Product Profile) and establishing clear “reasons to believe” that your product will both meet those needs and be attractive to users, technically feasible and commercially viable.
FEI in your development makes commercial sense, as it allows core thinking to be double checked and novel avenues to be explored when the cost of changing direction is lowest.
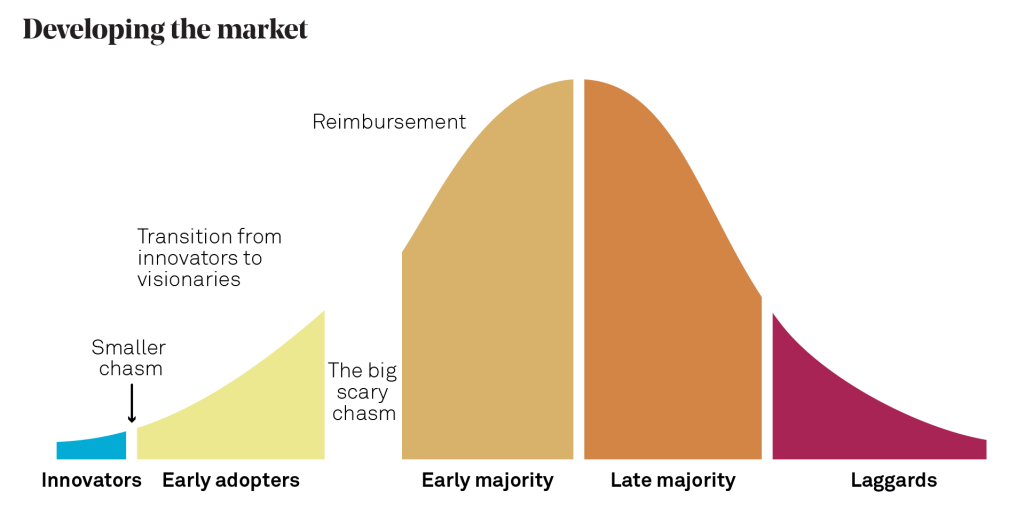
To help illustrate this, it is useful to consider the diagram below, which illustrates key stages of medical product commercialisation, namely developing the business, developing the product and developing the market. People who have read the book “Crossing the Chasm: Marketing and Selling High-Tech Products to Mainstream Consumers” by Geoffrey Moore will recognise that I am using his market adoption curve and famous “chasm” to represent the key market development stage.

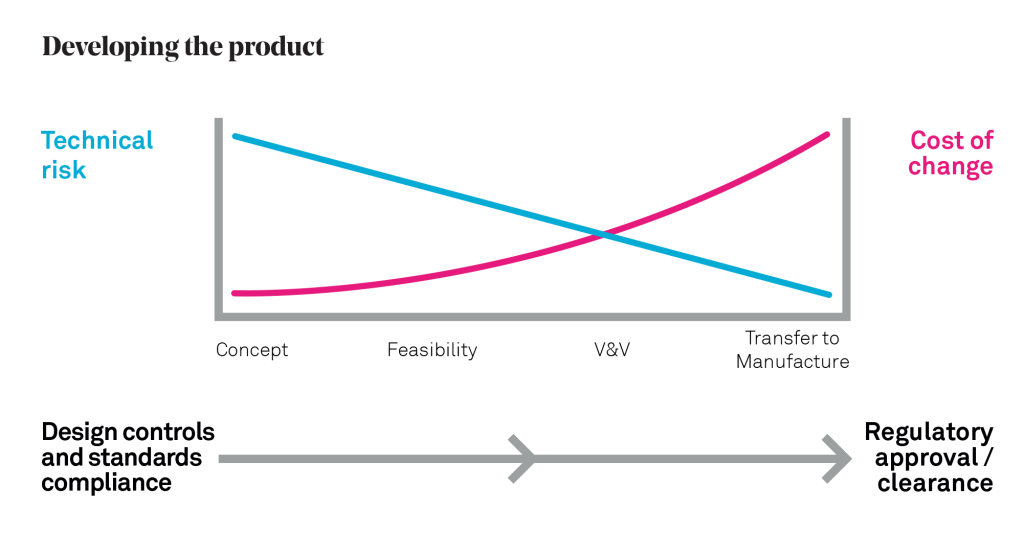
The key message to take away is that MedTech product design and development (the central section of the diagram) tends to be an expensive process. Technical requirements and constraints need to be solved and Design Control (as defined by section 7.3 in ISO 13485 and by FDA QSR 820.30) requires working to extensive QMS procedures and complying with multiple harmonised standards. As mentioned, just generating compliance documentation is a significant undertaking in itself. Recognising the scale of investment that will be required, and an escalating cost of change in the later stages of development, it makes a lot of sense to try to understand the market requirements as early as possible, and ideally before you have invested significantly in technical development.
Market adoption planning – understanding your commercial requirements
If we consider market adopters in the MedTech space, it might be that your innovators are clinician inventors, while early adopters are key opinion leaders at research hospitals (with the research budget and appetite to investigate novel technologies). The ‘chasm’ is the gap before your product is adopted as a standard of care across health systems. Often, this needs significant clinical data and reimbursement to allow uptake by the mass, early majority market.
By understanding the market adoption characteristics early, key requirements can be built into the design inputs at the start of product development. This is when cost of change is lowest - allowing for meaningful early concept work to be performed. It is important to really explore how it might be possible to realise MedTech products that are not just safe and effective, but also loved by end users and highly differentiated in a competitive market place.
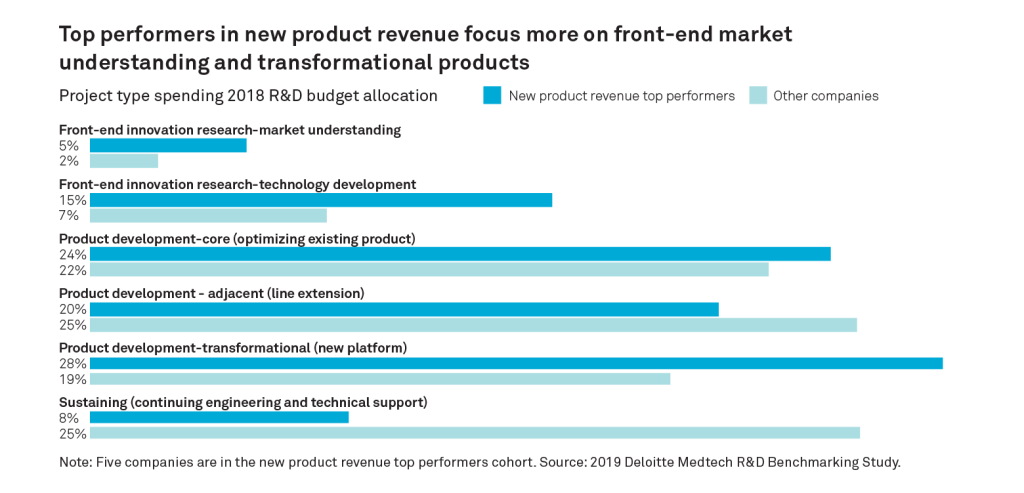
A report from the Deloitte Center for Health Solutions – “Benchmarking product development in MedTech – what high performing companies are doing differently”, nicely illustrates the value of investing in front end market understanding. The report shows that top MedTech performers allocated nearly twice the amount of investment in front-end market research compared to other companies. Digging deeper, it suggests that “top performers not only favour investing in market understanding early but also throughout the product development process”. These investments include both typical market research as well as active engagement with customers, product users and patients, to understand true unmet needs. This information can then be used as a valuable source of transformational product value.
Desirability
The pyramid below is a useful way to understand the concept of desirability (i.e. what makes sense or is attractive to users) in the MedTech product space. At the very base are the fundamental requirements of safety and effectiveness for end users. For medical products, conformance is achieved through the application of Human Factors Engineering (for example, see FDA-2011-D-0469) and Usability Engineering (see IEC 62366) which use a combination of user risk-related analysis and user studies (i.e. formative and summative studies) to deliver the minimum regulatory requirements of safety and effectiveness.
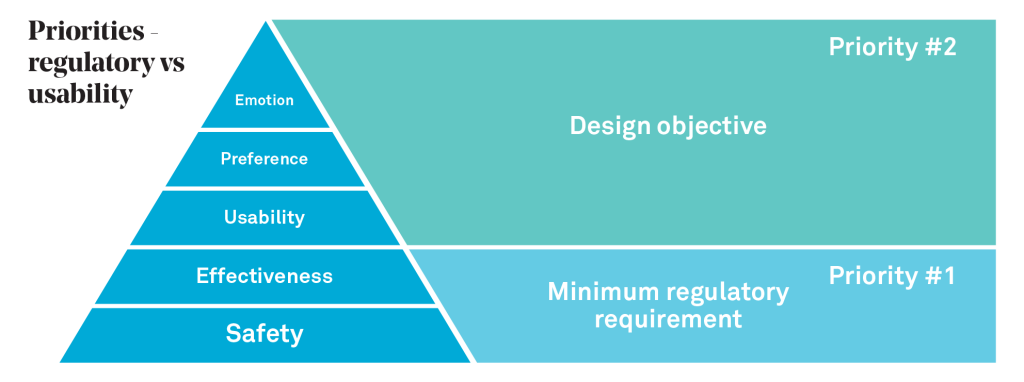
A safe and effective product is not necessarily a desirable product. However, ensuring safety and effectiveness does not necessarily ensure desirability and hence it is key to also understand the upper tiers of the pyramid, where the aim is to consider the emotional and practical mindset of the users. For example, how might we understand how a technology fits with someone’s lifestyle, particularly in light of illness and co-morbidities? What levers might exist to encourage behaviour change or a better fit with a user’s lifestyle?
Often, these deeper levels of insight are only achievable through the use of observational studies. In the book “Change by Design”, Tim Brown clearly illustrates the value that can be gained from observational studies:
“The basic problem is that people are so ingenious at adapting to inconvenient situations that they are often not even aware they are doing so; they sit on their seat belts, write their PINs on their hands, hang their jackets on doorknobs, and chain their bicycles to park benches. Henry Ford understood this when he remarked, “If I’d asked my customers what they wanted, they’d have said ‘a faster horse’.” This is why traditional techniques such as focus groups and surveys, which in most cases simply ask people what they want, rarely yield important insights. The tools of conventional market research can point to incremental improvements, but they will never lead to those rule-breaking, game changing, paradigm shifting break throughs that leave us scratching our heads and wondering why nobody thought of them before.”
Tim Brown identifies three key elements to inform design thinking: Insight (entering the environment and learning from the world of others), Observation (watching what people don’t do, listening to what they don’t say) and Empathy (standing in the shoes of others). These should all be considered in MedTech design.
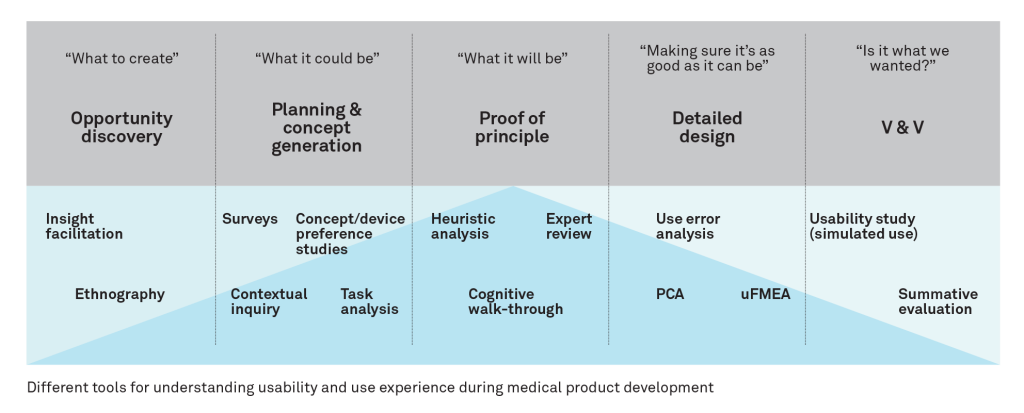
The key to gaining observational data as cost effectively as possible is to deploy the right tools at the right time in the development cycle. The diagram below illustrates the tools that Team often utilises for our clients at various stages in the development cycle.
Technical feasibility
Last up are the constraints related to technical feasibility, which in most cases mean constraints bounded by the laws of physics. It is no surprise that technical constraints can be expensive and time consuming to overcome in MedTech product design, and in some cases the laws of physics can be showstoppers.
When taking a harmonious approach to competing constraints, it can sometimes be more viable for technical constraints to be overcome by easing back on desirability and adopting a technical development ethos where form follows function. For example, in the case of miniaturising technologies, such as in the growing area of wearable devices, it can sometimes be that a small compromise in size or shape can significantly reduce your technical development risks related to thermal and power management. Nevertheless, when a client has the appetite and budget to take on technical risk, then some truly disruptive and inspirational products can emerge. In the case of LumiraDx, a client who set out to change the landscape of diagnostic testing by bringing laboratory level sensing to a point of care environment, the visionary output received high praise from Bill Gates, who said: “This diagnostic, the LumiraDx, is amazing. An innovation cheaper and smaller than the diagnostic devices that came before”.
At Team, we address technical feasibility from the outset. We create early-stage concepts and ensure that their selection and evolution is based on engineering judgements already focused on likelihood of success and the need for smooth transition to production. We use cutting edge prototyping techniques to review performance quickly and effectively, alongside utilising a wide range of analysis and modelling tools to challenge and build confidence in the technical feasibility of a design approach.
In all cases, we are seeking the most balanced approach to achieve effective results, at the appropriate resolution, in the most efficient manner. In some cases, for example with haemostat devices, where complex powder flow characteristics are in play, we may lean more towards rapid build/test/iterate loops than detailed analysis, because the analysis will be too complex and time consuming. The key for all developments, however, is to balance the use of empirical and analytical methods as appropriate.
It is also important to consider constraints related to manufacture. At Team, we advocate working closely with manufacturing partners as early as possible, in order to ensure that product designs are fully capable and realisable from fully validated processes. The key is to utilise tools and techniques such as Process FMEA and process mapping to identify critical failures as early as possible and to mitigate those failures before they occur.
Achieving balance
Balancing commercial viability, desirability and technical feasibility is a key focus for Team. Our project managers are trained to seek balance in whatever manner is best to meet the aspirations, risk appetite and budget of the clients we work with.
Sometimes, all focus might be on technical feasibility to confirm there are no showstoppers, or to demonstrate that challenging technical requirements can be met. In other cases, you may be seeking parallel tracks to more deeply understand commercial and desirability requirements, and minimise the risk of driving forward with expensive technical developments that do not meet real clinical or user needs.
In many cases there will also be conflicting constraints or requirements which need to be resolved. When this occurs, we recommend deferring to the safety critical nature of the industry and using both ISO 14971 and project risk management as the most effective method of arbitration.
Want more information on device development for MedTech start-ups? Request a free copy of our MedTech Handbook here.



