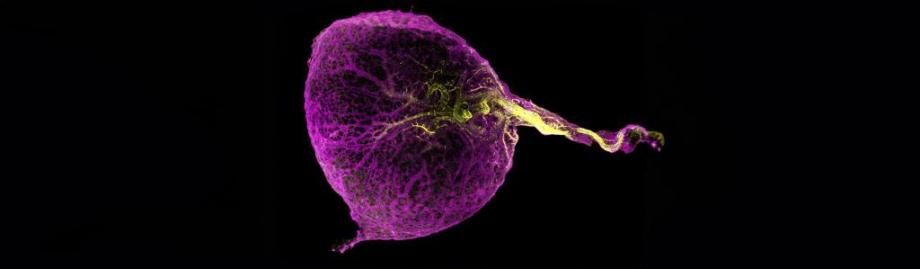
The role of the human yolk sac in supporting early embryonic development and the first wave of the prenatal immune system has been mapped in a study published today (17 August) in Science.
Researchers from the Wellcome Sanger Institute, Newcastle University, Cambridge Stem Cell Institute and collaborators discovered that the yolk sac has multiple organ functions - it acts like the liver to get rid of toxins and to make coagulation factors, as well as producing a key hormone - Erythropoietin (stimulates red blood cell production) that is normally produced by the kidney in adult life. It provides the functions performed by the liver, bone marrow and kidney before these organs are formed during early development. The study also shines a light on how blood and immune cells are produced within the yolk sac for the first time.
The yolk sac is externally connected to the embryo and develops within the uterus during the first weeks of pregnancy. It is vital for providing nutritional and metabolic support to the developing embryo.
This study is part of the international Human Cell Atlas* (HCA) initiative, which is mapping every cell type in the human body across the human lifespan, to transform our understanding of health and disease.
The work analysed ten yolk sac samples (1) and integrated external datasets enabling researchers to look at over 169,000 cells, spanning four to eight weeks after conception. By using cutting-edge single-cell sequencing (2), and whole organ imaging (3) of the human yolk sac, the researchers obtained a picture of the very start of the development of the immune system. This work is the final in a trilogy of papers that completes the analysis of immune system formation during gestation in three sites across the developing body (in the yolk sac, liver and bone marrow).
Many childhood diseases, such as leukaemias, have their origins in the early development of the immune system. However, most of what we know about early immune development has been inferred from animal studies, mostly in mice. Difficulty in accessing samples has hindered development studies in the past, restricting our understanding of the prenatal immune system.
The work presented today also uncovers a major finding - a new, accelerated way of producing macrophages very early in development. Researchers mapped how the first blood-producing stem cells emerge from blood vessel linings of the yolk sac. These stem cells produce different types of blood and immune cells in waves in the yolk sac and also make specialised immune cells called macrophages in an entirely different way from how they are made in adult life.
The macrophage development pathway appears unique to the early embryo - a rapid and direct route to get the cells the body needs. In contrast, later during development and in adult life stem cells make monocytes (an intermediate cell stage) which then transform into macrophages.
This finding could open the door to new and improved production of engineered macrophages with unique tissue-forming properties, which have many therapeutic applications, such as in regenerative medicine to heal wounds and in degenerative brain diseases such as Alzheimer’s disease.
Issac Goh, joint first author of the paper and visiting scientist at the Wellcome Sanger Institute, said: “This is the first time that the yolk sac has been profiled at a single cell level, giving us an incredible amount of information on how this primary organ works in the first stages of human development. It has given us novel insights into the earliest blood and immune cells we make, building on the work uncovered in previous studies from the Human Cell Atlas. We did not know that the yolk sac had these functions until now.”
Dr Rachel Botting, joint first author of the paper and visiting scientist at the Wellcome Sanger Institute, said: “It’s exciting to think of the possibilities stemming from this work, from new ways of artificially engineering cells in the lab to a better understanding of disease early on during pregnancy.”
Researchers also compared the human yolk sac data with rabbit and mouse yolk sac data and show that the human and rabbit yolk sac functions are similar and conserved, whilst the mouse yolk sac is different.
The human yolk sac is the predominant site for early red blood cell production, unlike in mice, where the liver is also an important contributor. This has implications for early development studies moving forward, showing that work on human tissue is critical to draw a clear picture of what happens during these initial phases.
Dr Laura Jardine, senior author of the study and group leader at Newcastle University, said: “Creating a comprehensive Human Atlas of blood and immune cell development in early life is an essential foundation to understanding what goes wrong in a wide range of diseases.”
Professor Muzlifah Haniffa, senior author and group leader at the Wellcome Sanger Institute, said: “Mapping out how the yolk sac evolves during these first weeks of pregnancy is fundamental to the understanding of the development of the immune system. This is the first time that we show the multiple organ functions of the yolk sac - we’ve seen a relay from the yolk sac to the liver, to the bone marrow. We’ve also discovered ways of learning how to produce cells that are different to those we produce in adult life. It means that cellular engineering doesn’t always have to follow the same method. Here's another recipe that gets you there faster.”
Image: courtesy of Wellcome Sanger Institute