I have myopia – near-sightedness. Naturally, this means I should have to be wearing glasses. However, most of my friends and colleagues have never seen me with them on before. This is because I have been wearing overnight orthokeratology contact lenses since the start of my diagnosis at age 11. These lenses have supported my vision without wearing any glasses or contact lenses during the day. More importantly, they have controlled the progression of my myopia. We often think of contact lenses simply as the more “aesthetic” alternative to spectacles for improving vision. However, there is more to contact lenses than meets the eye.
The following are some of the amazing applications of contact lenses, spanning drug delivery, condition management and health monitoring.
Drug delivery to the eye
Eyedrops are the most widely used form of ocular medication. Despite this, physiological and anatomical barriers of the eye mean that they only absorb an estimated 5% of the eyedrop. To really understand how a drug is delivered to the eye, we must first look at its structure.
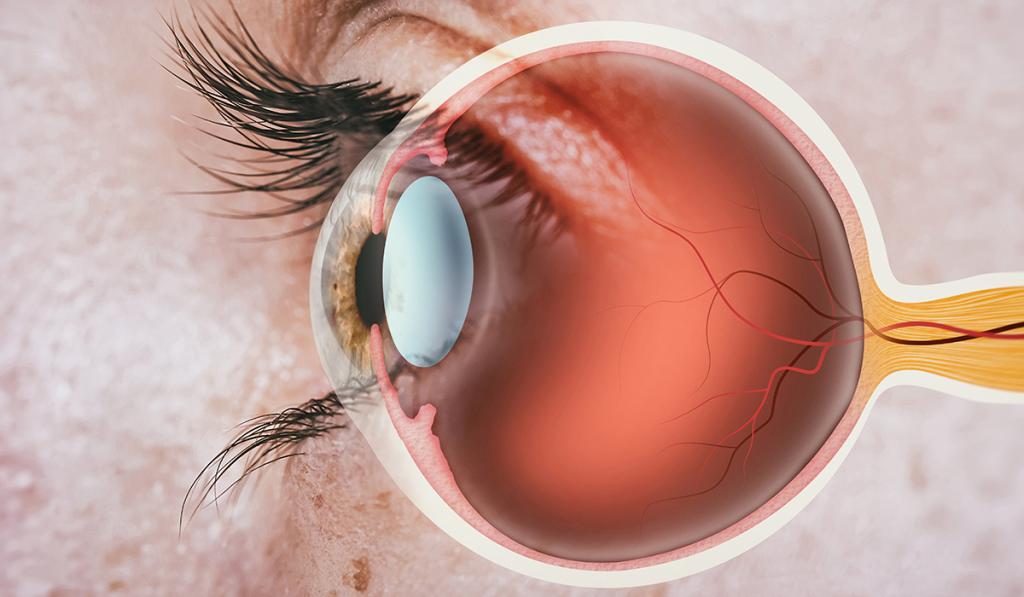
The two major barriers that prevent drugs from reaching the target site are the tear film and the cornea. Upon drug instillation, the medication mixes with the tear film, which is the first barrier. However, most gets drained away due to natural reflexes such as blinking and tearing. Moreover, the tear film is unable to contain all of the eyedrop since it only has a volume of about 3-10 microlitres which is significantly lower than the 25-70 microlitres in eyedrops. The second barrier is the cornea, which contains tight junctions that prevent foreign particles from entering the eye. As a result, the bioavailability (the rate at which drug enters the system) of eyedrops is low. This means patients need to apply eyedrops frequently, leading to low patient adherence.
Howerver, drug-eluting contact lenses are able to overcome the barrier posed by the tear film. The tear film is composed of three layers – the oily layer (lipid), watery layer (aqueous) and mucus layer (mucin). When contact lenses are placed in the eye, the mucus layer forms around the contact lens. Since this layer mixes poorly with the rest of the film, the drug can remain on the corneal surface for a longer period, giving the drug more time to reach the target site, increasing bioavailability to 50%.
Design challenges of drug-eluting contact lenses
There are a few challenges when designing drug-eluting contact lenses. The first is the compatibility between the structure and material of the lens and the drug. If the lens and drug are too compatible, the drug will bind to the lens instead of being released into the eye. The second is controlling the speed of drug release, which has been achieved through techniques such as nanoparticles and molecular imprinting. Lastly, regulations surrounding drug-eluting lenses might be trickier to navigate since they are combination products. They comprise of at least two regulated components: drug and medical device.
In early 2022, the FDA approved the first drug-eluting contact lens. This daily disposable lens provides relief for itchy eyes, which can be triggered by allergens such as dust and pollen. The novelty of the product is the compatibility between the drug Ketotifen and the material of the contact lens Etafilcon. Results show that users of the product experience relief in as fast as 15 minutes, for up to 12 hours.
Another company has developed an ink and printing process that allows an FDA-approved drug to be printed on an FDA-approved lens, allowing a sustained and continuous release of drug into the eye over a few days. A Phase 2a study in 2022 showed that patient tolerance to the imprinted lens for glaucoma treatment for over a period of eight days.
There are other drug-eluting contact lens currently in clinical trials to treat various ocular conditions such as ocular hypertension and cystoid macular oedema. With these breakthroughs, treatment for other eye diseases that require frequent use of eyedrops, such as Uveitis that may require the patient to use eye drops once every hour, can be transformed by drug-eluting contact lenses.
Managing ocular conditions
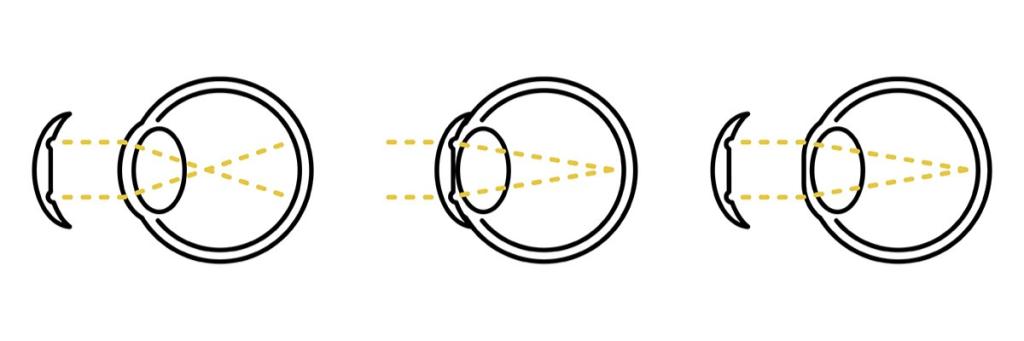
Orthokeratology contact lenses, or Ortho-K, are used to control myopia progression, especially in children. Myopia, or short-sightedness, results in blurred vision as light rays are focused in front of, instead of on, the retina. If not controlled, irreversible blindness will be inevitable due to changes in the eye structure with age.
Ortho-K lenses are worn during sleep to reshape the cornea, refocusing light on the retina to enable the possibility of clear vision during the day without the need to wear corrective lenses. The modification of the eye shape decreases the stimulus for eye growth, controlling myopia progression as a result. This technology is widely available and can provide an less invasive alternative to procedures such as Lasik.
A non-invasive treatment for Glaucoma
Besides myopia, it may now be possible to control glaucoma. Glaucoma is an ocular condition in which the optic nerve connecting the eye to the brain is damaged, leading to blindness. A common cause is fluid build-up in front of the eye, whereby the resulting pressure presses against and damages the optic nerve. Current treatments such as eyedrops and laser therapy can only slow down the progression of the disease.
Although eyedrops provide a non-invasive option it can take up to 8 weeks for the drops to take effect. Laser therapy can improve patient adherence but it is likely that the patient would need to undergo the procedure repeatedly as the fluid will continue to build up. One company has embedded a gold trace in the contact lens, which acts as an electrode. When used with a pair of glasses that generates a magnetic field, an electric current is induced in the trace. This current is then delivered to the drainage system of the eye, opening it and relieving ocular pressure as a result. Preliminary studies have shown an up to a 40% reduction in pressure within hours. Not only is this a non-invasive alternative that is potentially more effective than current treatments, it is unlikely to be considered a combination product, thereby avoiding more difficult regulatory hurdles.
Health monitoring via contact lenses
Health monitoring devices have multiple benefits, including collecting more data to observe trends, ensuring patient compliance and reducing routine check-ups. Recent developments in wearable devices include ‘smart contact lenses’ as biosensors for monitoring patient health, while in 2016 the FDA approved a contact lens that can monitor intraocular pressure for glaucoma treatment.
Studies have also presented the possibility of measuring glucose levels in tears. Having a contact lens with a glucose-measuring capability can be helpful for diabetic patients who need to prick their fingers to measure their glucose level. While some companies have faced challenges in developing such lenses, there are still ongoing developments in this area. For example, a research group is working towards smart photonic contact lenses that have dual functionality: glucose concentration analysis in tears using near-infrared light and drug delivery for retinopathy. The product also has Bluetooth capability to inform patients of their results.
These ongoing developments in health monitoring via contact lenses could have a number of potential benefits. For example, their inconspicuous nature could improve patient adherence, especially with conditions that require long periods of monitoring. Contact lenses are also a non-invasive method to collect data, offering potential relief to patients both physically and psychologically.
What’s next for ocular technologies?
These futuristic, smart contact lenses might seem like the stuff of movies, but they are not so far-fetched. As discussed, there are already various contact lenses with applications beyond just correcting vision that are FDA-approved and on the market. I am glad to have Ortho-K, but I do know that this treatment is not suited to all patients with the same visual impairment. Hence, it is exciting to see these ocular technologies develop. As technologies continue to evolve, we should set our sights on what else can be achieved with these ocular devices.
References
| M. Mofidfar, B. Abdi, S. Ahadian, E. Mostafavi, T. A. Desai, F. Abbasi, Y. Sun, E. E. Manche, C. N. Ta and C. W. Flowers, “Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies,” International Journal of Pharmaceutics, 2021. | |
| I. Rykowska, I. Nowak and N. Rafal, “Soft Contact Lenses as Drug Delivery Systems: A Review,” Molecules, 2021. | |
| R. Saabye and M. Jacobsen, “MISTGO – A New Microdosing Eye Medication Delivery System,” ONdrugDelivery, 11 March 2023. [Online]. Available: https://www.ondrugdelivery.com/mistgo-a-new-microdosing-eye-medication-delivery-system/. | |
| Editorial Team from Chronic Dry Eye, “Chronic Dry Eye: Parts and Function of Your Eyes and Tears,” Chronic Dry Eye, 19 April 2021. [Online]. Available: https://chronicdryeye.net/tears-function. | |
| S. Marx, J. Eckstein and W. Sickenberger, “Objective Analysis of Pre-Lens Tear Film Stability of Daily Disposable Contact Lenses Using Ring Mire Projection,” Objective Analysis of Pre-Lens Tear Film Stability of Daily Disposable Contact Lenses Using Ring Mire Projection, vol. 12, pp. 203-211, 2020. | |
| E. Mullin, “The First Drug-Releasing Contact Lens Is Here,” Wired, 30 March 2022. [Online]. Available: https://www.wired.com/story/the-first-drug-releasing-contact-lens-is-here/. | |
| “Johnson & Johnson Vision’s Investigational Antihistamine-Releasing Contact Lens Demonstrates Positive Phase 3 Results,” Johnson & Johnson, 26 March 2019. [Online]. Available: https://www.jjvision.com/press-release/johnson-johnson-visions-investigational-antihistamine-releasing-contact-lens. | |
| “Johnson & Johnson Vision Care Receives FDA Approval for ACUVUE® Theravision™ with Ketotifen – World’s First and Only Drug-Eluting Contact Lens,” Johnson & Johnson, 2 March 2022. [Online]. Available: https://www.jjvision.com/press-release/johnson-johnson-vision-care-receives-fda-approval-acuvuer-theravisiontm-ketotifen. | |
| B. Pall, Interviewee, First drug-eluting contact lens approved in Japan. [Interview]. 15 April 2021. | |
| “Contact Lens Drug Delivery Platform,” MediPrint Ophthalmics, [Online]. Available: https://mediprintlens.com/technology-science/. | |
| M. Barnett, “Contact Lens Drug Delivery Moves Closer to Reality,” [Online]. Available: https://na-prod-aventri-files.s3.amazonaws.com/html_file_uploads/2d5f8c7b3392bc6562add24a21eaad9e_Barnett_GSLS_poster_FINAL_1.7.2022.pdf?response-content-disposition=inline%3Bfilename%3D%22Barnett_GSLS_poster_FINAL_1.7.2022.pdf%22&response-content-type=ap. | |
| Study of LL-BMT1 in Patients With Elevated Intraocular Pressure, 2021. | |
| Therapeutic Contact Lens Drug Delivery System (TCL-DDS) in Patients With Recurrent Cystoid Macular Edema (ContactLens), 2020. | |
| Latanoprost Eluting Contact Lens for Treating Glaucoma and Ocular Hypertension, 2020. | |
| “Nearsightedness,” Mayo Clinic, [Online]. Available: https://www.mayoclinic.org/diseases-conditions/nearsightedness/symptoms-causes/syc-20375556#:~:text=Nearsightedness%20(myopia)%20is%20a%20common,to%20bend%20(refract)%20inaccurately. | |
| B. A. Holden, T. R. Fricke, D. A. Wilson, M. Jong, K. S. Naidoo, P. Sankarifurg, T. Y. Wong, T. K. Naduvilath and S. Resnikoff, “Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050,” Ophthalmology, vol. 123, no. 5, pp. 1036-1042, 2016. | |
| J. S. Harthan, “Overnight Orthokeratology for Myopia: What Does the Evidence Say?,” Review of Myopia Management, 3 June 2019. [Online]. Available: https://reviewofmm.com/overnight-orthokeratology-for-myopia-management-what-does-the-evidence-say/. | |
| NHS, “Glaucoma,” NHS, [Online]. Available: https://www.nhs.uk/conditions/glaucoma/#:~:text=Glaucoma%20is%20a%20common%20eye,not%20diagnosed%20and%20treated%20early. | |
| NHS, “Making a decision about Open-Angle Glaucoma,” [Online]. Available: https://www.england.nhs.uk/wp-content/uploads/2022/07/Making-a-decision-about-open-angle-glaucoma.pdf. | |
| C. Kent, “Treating with SLT First: The Pros and Cons,” Review of Ophthalmology, 9 June 2017. [Online]. Available: https://www.reviewofophthalmology.com/article/treating-with-slt-first-the-pros-and-cons. | |
| “Startup Developing New Glaucoma Treatment,” Inside INdiana Business, 14 November 2021. [Online]. Available: https://www.insideindianabusiness.com/videos/startup-developing-new-glaucoma-treatment. | |
| Purdue Research Foundation, “Purdue-affiliated startup developing non-invasive, effective contact lenses and glasses to treat glaucoma, prevent blindness,” Purdue Research Foundation, 31 May 2017. [Online]. Available: https://www.purdue.edu/newsroom/releases/2017/Q2/purdue-affiliated-startup-developing-non-invasive,-effective-contact-lenses-and-glasses-to-treat-glaucoma,-prevent-blindness.html. | |
| “Bionode: Jane Fischer — Global Innovations,” 2021. [Online]. Available: https://vimeo.com/502323679. | |
| Fda, “FDA permits marketing of device that senses optimal time to check patient’s eye pressure,” FDA, 4 March 2016. [Online]. Available: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-device-senses-optimal-time-check-patients-eye-pressure. | |
| X. Ma, S. Ahadian, S. Liu, J. Zhang and S. Liu, “Smart Contact Lenses for Biosensing Applications,” Advanced Intelligent Systems, vol. 3, no. 5, 2021. | |
| Photonics.com, “Diabetic Smart Contact Lenses Developed by South Korean Research Team,” Photonics Media, 15 January 2020. [Online]. Available: https://www.photonics.com/Articles/Diabetic_Smart_Contact_Lenses_Developed_by_South/a65468. | |
| “Importance of Proper Contact Lens Fitting,” Aspire Vision Care, [Online]. Available: https://www.aspirevisioncare.com/eyeglasses-contacts/contact-lenses/importance-of-proper-contact-lens-fitting/. | |
| “The EyePrint Impression Process: Easy, Painless, Precise.,” EyePrint Prosthetics, [Online]. Available: https://eyeprintpro.com/process/. | |
| H. Mirzajani, H. Mirlou, E. Istif, R. Singh and L. Beker, “Powering smart contact lenses for continuous health monitoring: Recent advancements and future challenges,” Biosensors and Bioelectronics, 2021. | |
| J. Park, J. Kim, S.-Y. Kim, W. H. Cheong and J. Jang, “Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays,” Science Advances, vol. 4, no. 1, 2018. |



