With the introduction of Medical Device Regulation [MDR] and In-Vitro Diagnostic Regulations [IVDR], the European Commission raises caution by emphasising traceability, patient safety and vigilance. This ‘cautioned emphasis’ can be seen in Medical Devices, Diagnostics and Digital Health regulatory requirements.
With the ongoing development of Software, technology, AI, ML and other technologies adopted by the MedTech industry, regulators and groups like the Medical Device Coordination Group [MDCG] are attempting to stay updated and provide manufacturers with suitable compliance guidance. As the industry is familiar with Software as a Medical Device [SaMD], a recent publication from MDCG requires Digital Health Companies to re-evaluate their products and familiarise themselves with Medical Device Software (MDSW) – Hardware combinations.
In October 2023, the MDCG published MDCG 2023-4 Medical Device Software (MDSW) – Hardware combinations - Guidance on MDSW intended to work in combination with hardware or hardware components.
This guidance document highlights
- Hardware or hardware component working in combination with MDSW
- External hardware component (e.g. sensor embedded in a dermal patch) providing input data to a MDSW app
- Hardware component incorporated within a smartphone or wearable connected to a MDSW app on smartphone or wearable
- Regulatory considerations
- Placement on the market
This guidance intends to examine and provide clarifications on which specific regulatory considerations apply when the hardware or hardware component incorporating the data collection element (camera, electrical/optical sensors etc.) are a ‘medical device’ or an ‘accessory to a medical device’. This guidance also outlines scenarios where the hardware or hardware component incorporating a data collection element are not medical devices or accessories to a medical device.
The guidance document highlights 4 different product scenarios where the Software utilises hardware to collect data, which is used for further diagnostics. Based on the intended purpose of the the Software and hardware, the guidance offers the following 3 options on how these products can be placed on the market :
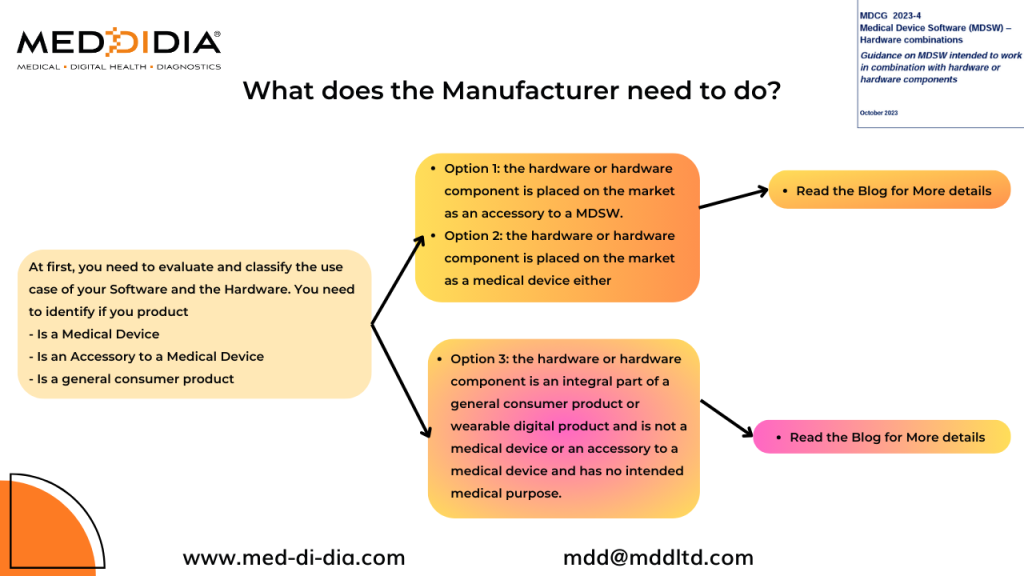
- Option 1: the hardware or hardware component is placed on the market as an accessory to a MDSW.
- Option 2: the hardware or hardware component is placed on the market as a medical device either
a) as part of a system according to article 22 MDR or
b) as a combination with another medical device according to article 2(1), or
c) as an integral part of a medical device.
- Option 3: the hardware or hardware component is an integral part of a general consumer product or wearable digital product and is not a medical device or an accessory to a medical device and has no intended medical purpose.
What does the Manufacturer need to do?
At first, you need to evaluate and classify the use case of your Software and the Hardware. You need to identify if you product
- Is a Medical Device
- Is an Accessory to a Medical Device
- Is a general consumer product
[analysis to see if your product fits into option 1, option 2, option 3]
Of course, it is always recommended that you consult a specialist when dealing with these products, which could also be classified as a borderline medical device. The Digital Health Team at Med-Di-Dia supports manufacturers with end-to-end regulatory compliance. Contact by sending an email to mdd@mddltd.com or visiting this link https://mailchi.mp/cef1e53ebb00/digitalhealth.
If your product is classified under option 1, option 2 [hardware or hardware component are qualified as either a medical device or an accessory to a medical device.] you must
- demonstrate compliance with the MDR
- follow general safety and performance requirements (GSPRs) mentioned in the MDR Annex I
- verify, validate and demonstrate the safety, reproducibility, compatibility and interoperability of the medical device or accessory to a medical device that the MDSW works in combination with, including all various configurations and variants
- Perform and fulfil Clinical Evaluation requirements
- Have a detailed Risk Management and Post-Market Surveillance plan where you must establish and implement appropriate communication channels to ensure efficient notification mechanisms regarding changes or incidents related to the medical device hardware, hardware component or accessory
If your product fits into option 3
- You are responsible for the safety, performance and reproducibility of the hardware or hardware component in its combined use with the MDSW in all intended configurations
- You must comply with the requirements under equivalent conditions to a situation where a manufacturer is combining a medical device with another product according to Article 22(4)
- Your product technical documentation must clearly identify and describe all other products (e.g., hardware or hardware component which are not qualified as a medical device or accessory to a medical device) that are intended to be used in combination with it
- You must demonstrate clinical evidence for all intended configurations (e.g., all platforms the MDSW is running on)
- Unlike the products classified under Option 1 and Option 2, all products classified under Option 3 have stricter and exhaustive Risk Management and Post-Market Vigilance requirements
The European Regulatory Environment continues to be a maze where the navigation requires proper ‘tips, tricks and spells’. The Digital Health Team at Med-Di-Dia can help manufacturers cut through the maze with end-to-end regulatory compliance. Contact by sending an email to mdd@mddltd.com or visiting this link https://mailchi.mp/cef1e53ebb00/digitalhealth.



